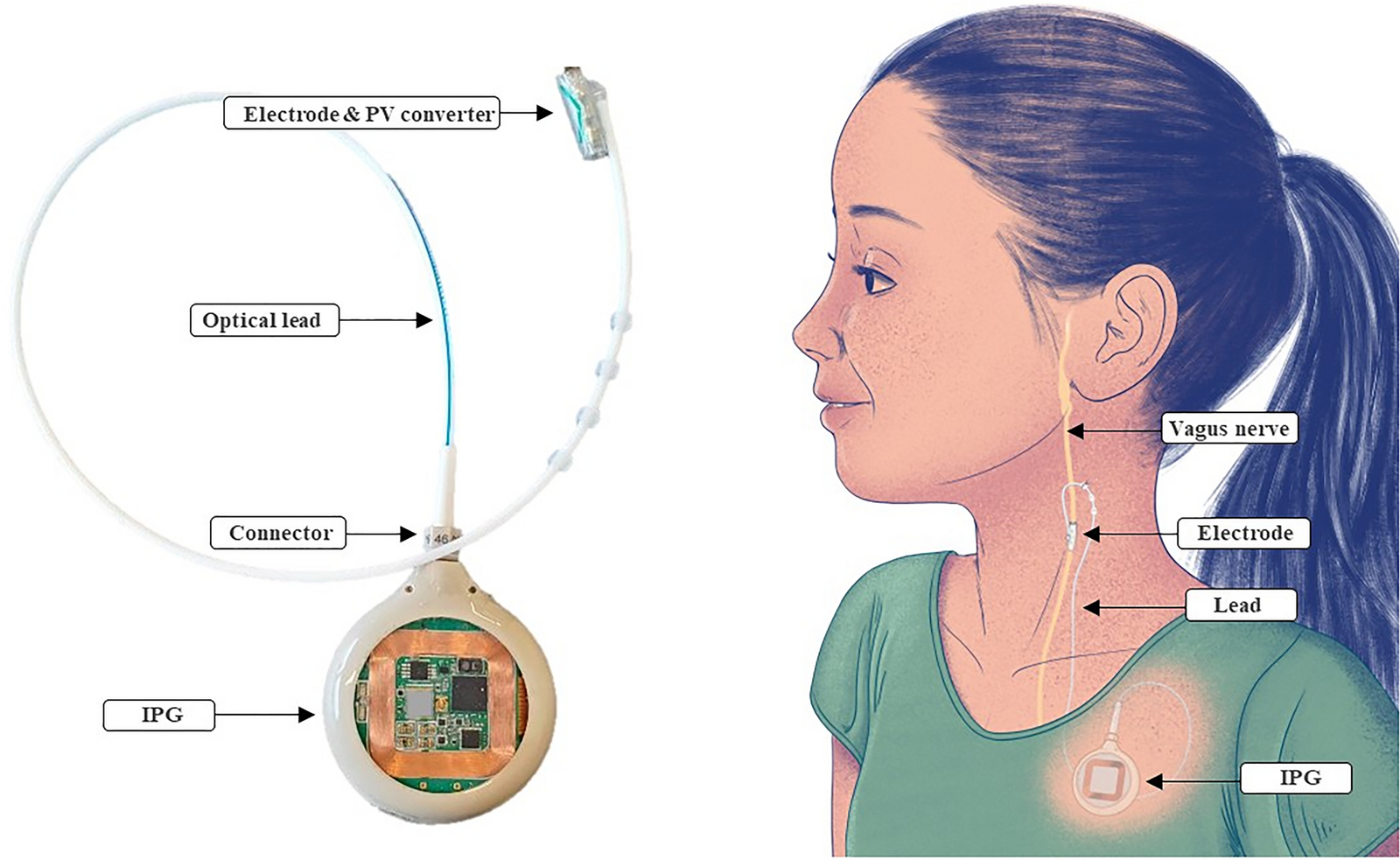
Non-Invasive Neurostim Contraindications: What You Must Know
Non-invasive neurostimulation devices have become powerful allies in the battle against insomnia, depression, chronic pain, and cognitive fog. From Apollo Neuro to NeuroVIZR, these wearable solutions are designed to calm, focus, and rebalance your brain. But here’s what rarely gets discussed—who shouldn’t use them.
At NeuroTechInsider.com, we’re all about testing, reviewing, and decoding the science behind these innovations. But we also want you to use them safely and wisely. If you’re considering devices like CES headsets, tDCS kits, or vagus nerve stimulators, this guide unpacks the contraindications of non-invasive neurostimulation you need to know before you even flip the switch.
Understanding Non-Invasive Neurostimulation
What Is Non-Invasive Neurostimulation?
Non-invasive neurostimulation refers to any device that modulates your brain or nerve activity from the outside of your body—no implants, no surgeries. These devices use either magnetic fields, low electric current, or sound/light pulses to interact with the nervous system.
- TMS (Transcranial Magnetic Stimulation): Uses magnetic fields to trigger neuronal activity.
- tDCS (Transcranial Direct Current Stimulation): Sends mild electric current through the skull to alter brain function.
- nVNS (Non-invasive Vagus Nerve Stimulation): Stimulates the vagus nerve via the neck or ear for autonomic rebalancing.
Devices like Sensate, Audicin, and Apollo Neuro fall under this umbrella, though they differ in their mechanisms and intensity levels.
Popular Use Cases
- Relief for anxiety, ADHD, PTSD
- Insomnia and circadian rhythm support
- Focus enhancement and executive function
- Mood regulation and burnout recovery
“These technologies offer real promise—but only if they’re matched with the right person, at the right time, and with the right precautions.”
Why Contraindications Matter
Think Safety First, Stimulation Second
While most devices are non-invasive and FDA-cleared, neurostimulation still modifies your nervous system. Without proper screening, it can cause unintended consequences. The most serious? Seizures, syncope, arrhythmias, or device malfunction.
One study from MedRxiv (2023) highlighted neural overstimulation risks in patients with hidden contraindications. Here’s a snapshot from their report:
Contraindications Aren’t One-Size-Fits-All
Contraindications vary by device type and by your personal health profile. Some are absolute (never use the device), others are relative (only under supervision). The key is to screen thoroughly before you begin.
General Contraindications
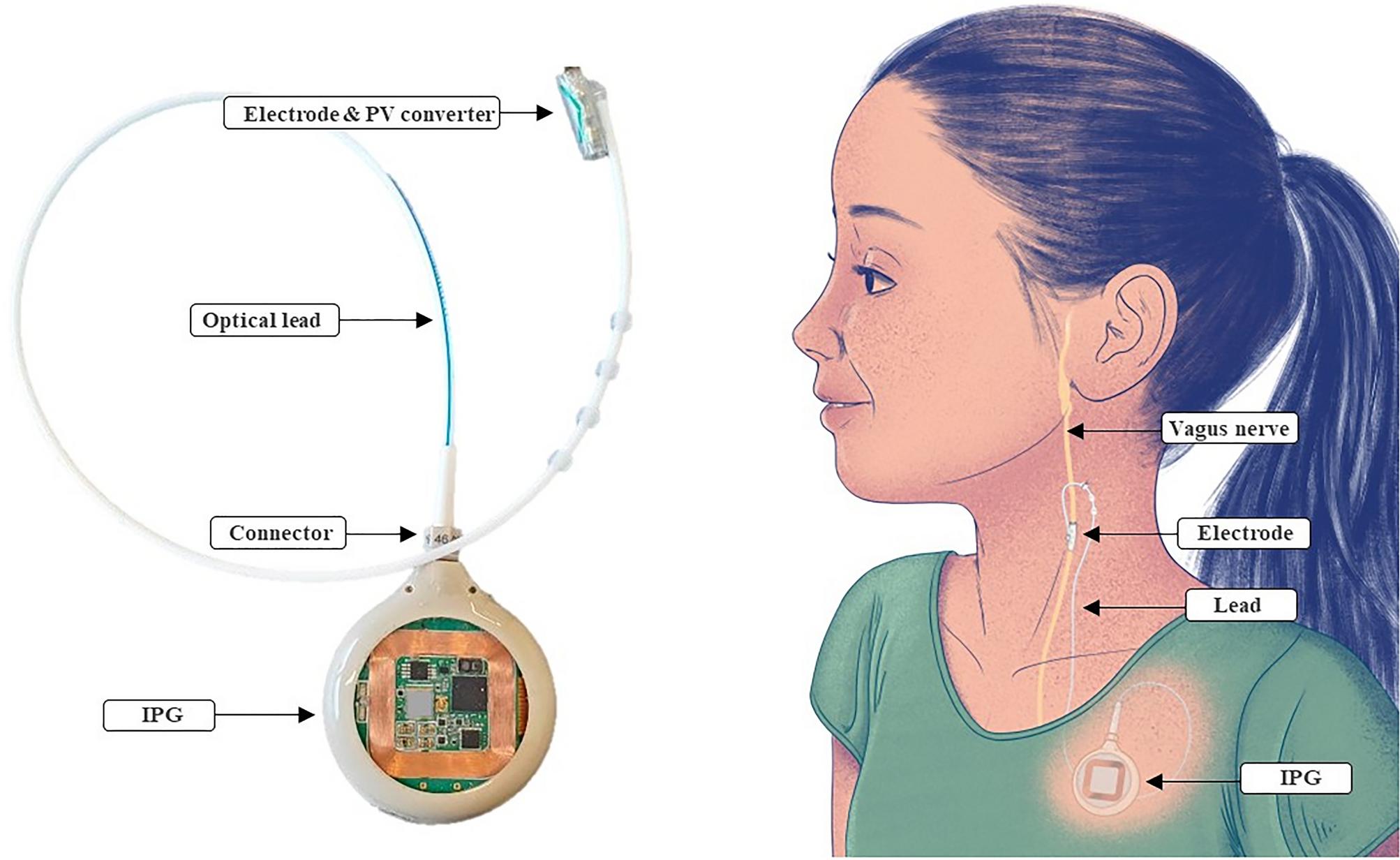
1. Implanted Medical Devices
Electromagnetic stimulation can interfere with implanted electronics, causing them to misfire or malfunction. That includes:
- Pacemakers and defibrillators
- Cochlear implants
- Deep brain stimulators
- Vagal nerve stimulators
- Metallic implants in the skull or neck (excluding titanium)
See this MRI-based analysis from Springer on magnetic interference potential:
2. Metallic Foreign Bodies
If you’ve got metal fragments in your head or face—whether from past surgery, military service, or accidents—think twice.
- Cranial metal fragments or shrapnel
- Steel aneurysm clips or ocular implants
- Dental bridges with magnetic components
Even minor electromagnetic fields from tDCS or TMS can provoke localized heating or nerve reactivity.
3. Pregnancy
While there’s no confirmed harm, most device manufacturers and clinicians advise avoiding neurostimulation during pregnancy due to insufficient safety data. That includes all three categories—TMS, tDCS, and nVNS.
4. History of Seizures or Epilepsy
If you or your immediate family has a history of seizures, proceed with extreme caution. Devices like TMS have been known to induce seizures in rare cases—especially when used at high frequencies or in epileptogenic zones.
According to the Practical Neurology Journal:
“Even low-level devices may pose seizure risks when users have underlying neurological instability.”
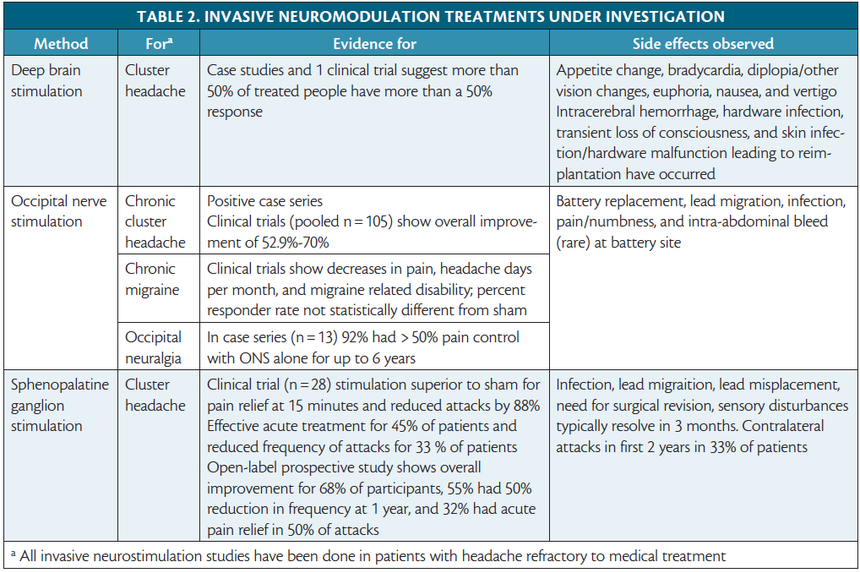
5. Serious Head Trauma or Neurosurgery
Have you had brain surgery or traumatic brain injury? Your brain’s electrical conductivity may be altered, making it less predictable under stimulation. Always consult a neurologist before trying neurotech tools like tDCS or AVE headsets.
6. Skin or Scalp Conditions
Stimulation pads and sensors need to sit on clean, intact skin. If you have:
- Open wounds
- Active eczema or psoriasis
- Recent burns or inflammation
…you should avoid placing stimulation pads over those areas. Skin irritation is one of the most common—but preventable—side effects.
Coming Up: Device-Specific Warnings, Medications & Age Limits
In the second half of this guide, we’ll break down:
- Risks unique to TMS, tDCS, and nVNS
- How prescription drugs can amplify risks
- Why most devices aren’t cleared for kids and teens
- How to do a comprehensive safety screening at home
Stay with us at NeuroTechInsider.com—your inside track on the future of drug-free, device-led brain wellness.
Device-Specific Contraindications
Not all neurostimulation devices carry the same risks. Some interact with the brain’s electrical circuits, others modulate peripheral nerves or sensory systems. Here’s what you need to know about contraindications specific to the most common technologies.
TMS (Transcranial Magnetic Stimulation)
- Metallic cranial implants – Especially non-titanium metals
- History of epilepsy or seizures – Even controlled cases warrant extreme caution
- Pregnancy – Limited data exists on fetal effects
- Severe head trauma or brain surgery
Because TMS uses high-intensity magnetic fields, it’s best administered in a clinical setting with EEG monitoring when used on high-risk individuals. If you’re curious about TMS for home use, read our breakdown of TMS alternatives designed for safer DIY stimulation.
tDCS (Transcranial Direct Current Stimulation)
- Skin lesions or open wounds at the electrode site
- Metal in skull (e.g., surgical plates)
- Epilepsy or seizure disorders
- Children under 16 – most devices are not cleared
tDCS is widely available in consumer-grade devices, but improper use—especially at high amperages or long sessions—can increase seizure risk or cause persistent fatigue. Always follow dosage protocols from clinical literature or expert-reviewed device manuals.
nVNS (Non-Invasive Vagus Nerve Stimulation)
- Implanted electronic devices (pacemakers, defibrillators)
- Cardiac conduction disorders
- Bilateral vagotomy – nerves removed or damaged
While devices like Sensate and Apollo Neuro offer gentle nVNS-style stimulation through vibrations and infrasonic feedback, stronger nVNS devices may trigger unintended responses in people with heart or nerve conditions.
Age and Medication Considerations
Children and Adolescents
Most neurostimulation devices have not been approved for use in individuals under 16 or 18 years old, depending on the manufacturer. The adolescent brain is still developing, and stimulation may have unpredictable effects on cognition, neuroplasticity, and emotional regulation.
Instead of electrical stimulation, consider light-sound therapy or neurofeedback tools which are gentler and less invasive.
Medications That May Amplify Risks
Some prescriptions and OTC medications can lower the seizure threshold or interact poorly with neurostim devices, including:
- Tricyclic antidepressants
- Antipsychotics (e.g., risperidone, olanzapine)
- Benzodiazepines
- Stimulants for ADHD
- Antihistamines in high doses
Pro tip: Always list all medications—prescribed, herbal, and recreational—when consulting a clinician before using neurostimulation tools.
Comprehensive Pre-Treatment Screening
Start With a Medical History Review
Before using any neuro-enhancement device, ask yourself:
- Do I have a pacemaker or any surgical implants?
- Have I ever had seizures or neurological trauma?
- Am I on medications that affect my brain or nervous system?
- Do I have skin issues at the electrode/stimulation site?
If you answered yes to any of the above, consult your doctor before proceeding. Even “mild” consumer devices can compound hidden risks.
Follow Device-Specific Guidelines
Every reputable neurotech brand—whether it’s NeuroVIZR, NR9, or others—publishes safety guidelines. Read them. Understand what they recommend—and what they don’t.
Summary Table: Contraindication Categories
| Contraindication Category | Examples |
|---|---|
| Implanted Devices | Pacemaker, cochlear implant, DBS, metallic skull plates |
| Metallic Foreign Bodies | Cranial fragments, magnetic dental hardware |
| Neurological History | Seizures, epilepsy, brain surgery, trauma |
| Skin Conditions | Wounds, eczema, psoriasis at stimulation site |
| Medications | Seizure-threshold lowering drugs |
| Age | Under 16 or 18 (device-specific) |
Conclusion: Stay Safe, Stay Informed
Neurostimulation is one of the most promising fields in modern wellness. It’s non-pharmacological, highly customizable, and often fast-acting. But like all powerful tools, it must be used with discernment.
If you’re considering a neurotech device—be it for sleep, anxiety, burnout, or focus—start by understanding your own physiology. Then match that to a product that suits your needs and medical profile.
“The best neurotech is the one that’s safe, smart, and built for your unique brain.”
Explore our curated reviews and science-backed guides at NeuroTechInsider.com, your go-to hub for the safest, smartest, and most effective neurostimulation tech on the market today.
Frequently Asked Questions (FAQs)
❓ Can I use a neurostim device if I have migraines?
Yes, but only certain devices like CES and nVNS are suitable. Avoid TMS unless clinically prescribed. Always consult a neurologist.
❓ What if I have dental implants?
Titanium implants are generally safe. However, magnetic or metallic prosthetics in the cranial region may pose risks with TMS or tDCS.
❓ Is light-sound therapy safer than TMS or tDCS?
Generally, yes. Devices like Audicin or NeuroVIZR use frequency entrainment rather than electric/magnetic currents, making them gentler but still effective.
❓ How do I know if a device is FDA-cleared?
Look for “FDA-cleared” on the product box or documentation. You can also search the FDA medical device database directly.