Sleep Technology Safety Certifications: Full Guide for 2025
At NeuroTechInsider.com, we’re committed to bringing you the most trusted, data-backed reviews of non-invasive sleep and brain wellness technologies. But beyond features and pricing, there’s a crucial layer every biohacker, clinician, or wellness junkie needs to understand: sleep technology safety certifications.
Whether you’re eyeing a NeuroVIZR for cognitive uplift or exploring an FDA-cleared neurostimulator for sleep apnea, the right certifications ensure these devices do what they claim—safely and effectively.
TL;DR: If it tracks, stimulates, or regulates your sleep, it better be certified. Let’s break down the FDA approvals, CE markings, ISO standards, and what they mean for your health and wallet.
Why Safety Certifications Matter in Sleep Tech
The sleep tech space is booming with innovation—from wrist-worn trackers to smart headbands that pulse low-frequency stimulation. But innovation without regulation can be dangerous. Here’s why certifications matter:
- Health risks: Non-certified devices can emit unsafe EMFs, cause skin burns, or deliver inaccurate data that delays medical diagnosis.
- Legal protection: Certified devices are tested for liability and post-market surveillance. If something goes wrong, you have recourse.
- Clinical validity: Certifications like the FDA 510(k) confirm the device’s claims with clinical evidence, not just hype.
Still think your imported knockoff vagus stimulator is a good deal?
Key Certification Bodies & Standards by Region
1. United States: FDA Certification Explained
The U.S. Food and Drug Administration (FDA) oversees medical devices under the 510(k) clearance process. This is essential for sleep-related medical devices such as:
- Home Sleep Apnea Testing (HSAT) kits
- Implanted neurostimulators for central sleep apnea
- Closed-loop brainwave modulation wearables
For example, the remedē® System by Respicardia is a fully implanted neurostimulator that treats central sleep apnea—FDA cleared and clinically validated.
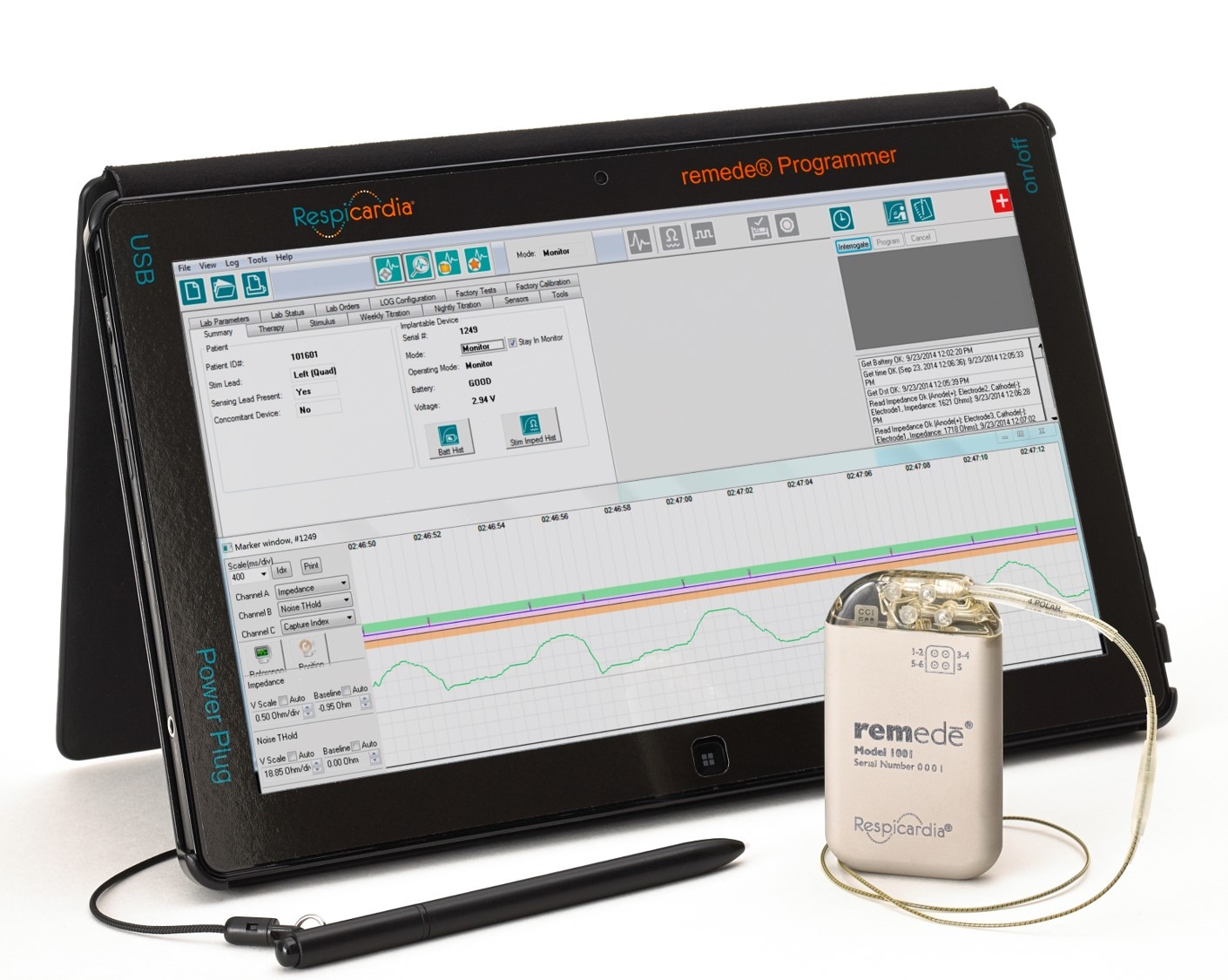
What’s involved in 510(k) approval?
- Substantial equivalence: Show your device works as well as something already FDA-approved.
- Clinical trials: Required for new tech or novel claims.
- Biocompatibility & electrical safety testing: Especially for devices in contact with skin or near the brain.
Heads up: If a company markets a device with medical claims (like “treats insomnia” or “diagnoses apnea”) but isn’t FDA-cleared, that’s a red flag.
Important: Consumer wearables like Fitbit, Oura, and NeuroVIZR don’t need FDA approval unless they’re intended for diagnosis or treatment.
2. Europe: CE Marking and MDR Compliance
In the EU, sleep tech is regulated under the Medical Device Regulation (MDR 2017/745). Devices intended for medical use—say a headband that delivers neuromodulation—must obtain the CE mark.
How CE marking works:
- Class I devices (e.g., basic monitors): Self-certified with a technical file.
- Class IIa/IIb/III (e.g., sleep neurostimulators): Require assessment by a notified body.
- Documentation includes safety tests, clinical evaluation, and usability validation.
Take for example, CE-certified HSAT kits—like the ones sold across the UK, Ireland, and EU sleep clinics.

Want to verify a CE mark? Look for the 4-digit notified body number next to the CE symbol and search their database.
3. Global Certifications: ISO, IEC & Cybersecurity
Outside of the U.S. and EU, sleep tech companies follow internationally recognized standards to build safe and compliant devices. The most important ones?
- ISO 10993: Biocompatibility for devices that touch skin or mucosa.
- IEC 60601-1: Electrical safety of medical devices.
- IEC 60601-1-2: Electromagnetic compatibility—essential for wearable devices.
- ISO 62304: Software lifecycle process, especially for AI-integrated sleep tech.
- UL 2900: Cybersecurity requirements for devices with wireless or app connectivity.
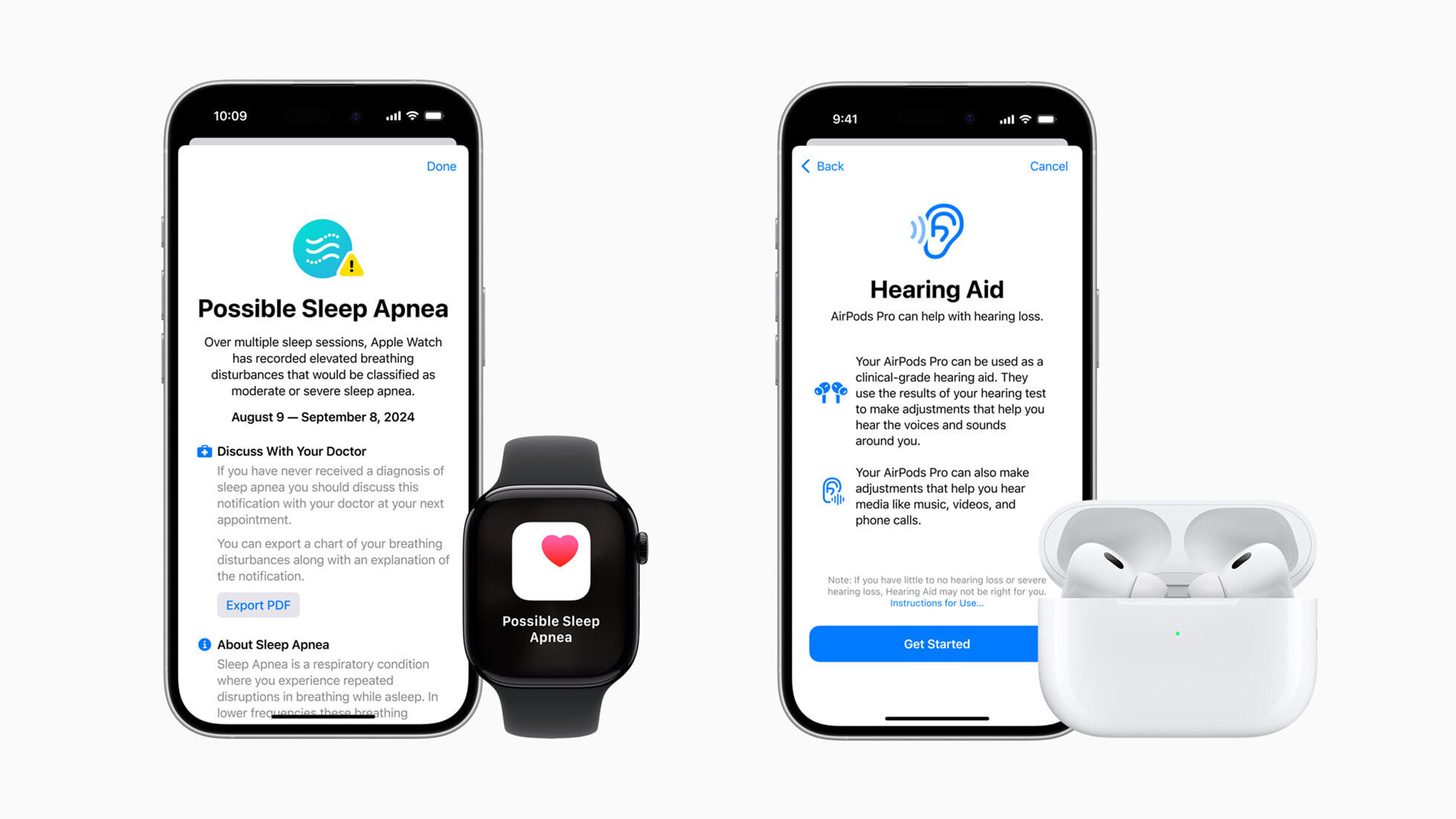
Global companies like Apple—soon to roll out sleep apnea detection via Apple Watch—must align with both U.S. and global standards. Expect devices like NeuroVIZR and Apollo Neuro to increasingly adopt ISO and IEC compliance as they expand into clinical use cases.
Next up: We’ll cover professional sleep lab certifications (AASM, BRPT), consumer product safety standards, and how YOU can verify a device before buying it. Plus, a practical checklist for manufacturers and biohackers alike.
Continue reading →
⚕️ 4. Professional Accreditation: AASM, BRPT, ESRS
Not all safety certifications are about devices. Sleep technology is also deeply tied to human operators—technologists, clinicians, and labs. That’s where professional accreditation comes in.
The American Academy of Sleep Medicine (AASM) sets the gold standard for U.S. sleep labs. If a clinic is AASM-accredited, it means their environment, processes, and equipment meet strict protocols for clinical sleep diagnostics.
- BRPT certifies technologists—look for RPSGT or CPSGT credentials.
- ESRS does the same across Europe, ensuring standardized competency across borders.
- Labs must meet protocols around safety, equipment hygiene, and EEG calibration.
For devices like Audicin or NR9 that get deployed in sleep clinics or neurofeedback centers, using them in an accredited facility increases patient trust and clinical validity.
“If a wearable is used in AASM-accredited studies, that’s a strong indicator it’s more than just wellness marketing.”
️ 5. Consumer Product Safety & Wellness Tech Compliance
What about devices that don’t diagnose or treat—like NeuroVIZR, Apollo Neuro, or Sensate? These fall under **wellness tech**, and although they’re exempt from FDA/CE medical approvals, they still need to pass **general safety standards**:
- Oeko-Tex®: Textile safety (non-toxic materials, no off-gassing)
- ASTM mechanical tests: Ensures devices won’t pinch, burn, or break under normal use
- California Proposition 65: Requires warning labels for any known carcinogens or toxins
- UL electrical safety: Applies to chargers, batteries, and devices worn near the heart or head
Consumer-grade neurotech must also comply with rules on labeling, instruction manuals, and use-case disclaimers. If the device is for kids or seniors, even stricter oversight applies.
⚠️ Red flag: If your wearable stimulator doesn’t list any safety standards, doesn’t come with clear instructions, and has zero transparency around testing—skip it. No matter how cool it looks on TikTok.
How to Get Certified: For Founders & Biohackers
If you’re developing the next-gen light-sound therapy headset or an AI-driven sleep neurostimulation band, here’s what it takes to become legit:
- Decide your category: Medical device or wellness product? That determines your entire regulatory path.
- Hire a regulatory consultant. It’s not optional unless you want to spend years lost in compliance jargon.
- Document everything: hardware specs, risk assessments, test reports, software lifecycle files (for ISO 62304).
- Engage with a Notified Body (EU) or submit to the FDA 510(k) program (U.S.)
- Perform usability studies and clinical validation (for medical claims).
And here’s a pro tip: Even if your product isn’t medical, aligning with ISO/IEC standards builds trust and boosts your sales pitch to clinicians and wellness centers alike.
Certification Comparison Table
| Region | Standard/Body | Applies To | Key Requirements |
|---|---|---|---|
| USA | FDA 510(k) | Medical sleep devices | Clinical trials, biocompatibility, performance |
| EU | CE Mark (MDR) | Medical devices | Risk classification, Notified Body approval |
| Global | ISO 10993, IEC 60601, UL 2900 | All sleep/neuro devices | Biocompatibility, EMC, cybersecurity |
| Labs | AASM, BRPT, ESRS | Sleep technologists & clinics | Facility protocols, staff certification |
| Consumer | ASTM, Oeko-Tex, Prop 65 | Wellness devices | Materials safety, mechanical testing |
How Consumers Can Verify Certification
Before you drop hundreds on a new neuro-enhancement wearable, do your homework. Here’s how to verify its legitimacy:
- Search the FDA Medical Device Database
- Check for CE mark & 4-digit Notified Body ID
- Request test reports or certificates from the brand (if not publicly available)
- Look for peer-reviewed studies using the device
- Scan for reviews from clinicians, not just influencers
At NeuroTechInsider.com, we include certification status and safety testing info in every product review—because your brain deserves more than marketing fluff.
♀️ Frequently Asked Questions
Do all sleep wearables need FDA approval?
No. Devices marketed for general wellness—like stress relief, mood improvement, or sleep tracking—don’t need FDA clearance. Only those that claim to diagnose or treat medical conditions do.
Is CE marking enough for safety?
It depends. For medical claims in the EU, yes. But wellness tech with CE marking still needs to comply with consumer product safety regulations. CE alone doesn’t mean the device is clinically validated.
What does ISO 10993 mean?
It’s a biocompatibility standard—proving that the materials used in your wearable are safe for contact with human skin or tissue. Critical for headbands, wrist wearables, and neurostimulators.
How do I report a problem with a device?
If the device is FDA-registered, you can report adverse events through FDA MedWatch. For CE-marked devices, contact the manufacturer and relevant Notified Body.
Final Thoughts on Sleep Tech Safety
Innovation in sleep and neurotech is accelerating. But with powerful brain-targeted tech comes responsibility. Whether you’re buying your first 40Hz headset or launching the next NeuroVIZR competitor, understanding sleep technology safety certifications is the key to staying legal, safe, and scientifically credible.
Always choose devices that are certified, clinically validated, or transparently tested.
At NeuroTechInsider.com, we’re building the go-to knowledge base for non-invasive brain enhancement devices. Follow us for hands-on reviews, expert comparisons, and the hard questions most influencers skip.
No hype. Just neuroscience and safety-first reviews for the next-gen brain tech revolution.